Written by: AIHC Blogger
This educational article is based on clinical information as of the publication date and primarily based on government and coding guidelines to assist coders, billers, clinical documentation improvement professionals and auditors address appropriate documentation for filing claims in the United States.
Overview of the U.S. Monkeypox Outbreak
Monkeypox is a rare disease caused by infection with the monkeypox virus. Monkeypox virus is part of the same family of viruses as variola virus, the virus that causes smallpox. Monkeypox symptoms are similar to smallpox symptoms, but milder, and monkeypox is rarely fatal. Monkeypox is not related to chickenpox.
Despite being named “monkeypox,” the source of the disease remains unknown. The first human case of monkeypox was recorded in 1970. Prior to the 2022 outbreak, monkeypox had been reported in people in several central and western African countries. Previously, almost all monkeypox cases in people outside of Africa were linked to international travel to countries where the disease commonly occurs or through imported animals. These cases occurred on multiple continents.
The monkeypox virus is spreading mostly through close, intimate contact with someone who has monkeypox. Click Here for more information from the Centers for Disease Control and Prevention (CDC) regarding how this virus spreads. The CDC is urging healthcare providers in the United States to be alert for patients who have rash illnesses consistent with monkeypox.
CDC is tracking an outbreak of monkeypox that has spread across several countries that don’t normally report monkeypox, including the United States.
Tracking Cases in U.S. and Globally
These hyperlinks provide a current U.S. Map & Case Count as well as a Monkeypox outbreak Global Map reporting the CDC’s tracing of cases.
2022 U.S. Map & Case Count
Monkeypox Outbreak Global Map
Monkeypox Case Definitions
A case may be excluded as a suspect, probable, or confirmed case if:
- An alternative diagnosis can fully explain the illness OR
- An individual with symptoms consistent with monkeypox does not develop a rash within 5 days of illness onset OR
- A case where high-quality specimens do not demonstrate the presence of Orthopoxvirus or Monkeypox virus or antibodies to orthopoxvirus
Clinical suspicion may exist if presentation is consistent with illnesses confused with monkeypox, such as secondary syphilis, herpes, and varicella zoster.
Suspect Case
A suspected case is when a patient presents with a new characteristic rash or meets one of the epidemiologic criteria and has a high clinical suspicion for monkeypox.
Epidemiologic Criteria - Within 21 days of illness onset:
- Reports having contact with a person or people with a similar appearing rash or who received a diagnosis of confirmed or probable monkeypox OR
- Had close or intimate in-person contact with individuals in a social network experiencing monkeypox activity, this includes men who have sex with men (MSM) who meet partners through an online website, digital application (“app”), or social event (e.g., a bar or party) OR
- Traveled outside the US to a country with confirmed cases of monkeypox or where Monkeypox virus is endemic OR
- Had contact with a dead or live wild animal or exotic pet that is an African endemic species or used a product derived from such animals (e.g., game meat, creams, lotions, powders, etc.)
Probable Case
A probable case is defined when there is no suspicion of other recent Orthopoxvirus exposure (e.g., Vaccinia virus in ACAM2000 vaccination) AND demonstration of the presence of:
- Orthopoxvirus DNA by polymerase chain reaction testing of a clinical specimen OR
- Orthopoxvirus using immunohistochemical or electron microscopy testing methods OR
- Demonstration of detectable levels of anti-orthopoxvirus IgM antibody during the period of 4 to 56 days after rash onset
Confirmed Case
A confirmed case should have documentation to demonstrate the presence of Monkeypox virus DNA by polymerase chain reaction testing or Next-Generation sequencing of a clinical specimen OR isolation of Monkeypox virus in culture from a clinical specimen.
The Infection
Infection with monkeypox virus begins with an incubation period which is roughly 1-2 weeks. A person does not have symptoms and may feel fine. The CDC states that a person is NOT contagious during the incubation period.
The illness typically lasts 2-4 weeks. The severity of illness can depend upon the initial health of the individual, the route of exposure, and the strain of the infecting virus.
Monkeypox infections are similar to the clinical course of ordinary discrete smallpox. A feature that distinguishes infection with monkeypox from that of smallpox is the development of swollen lymph nodes (lymphadenopathy). Swelling of the lymph nodes may be generalized (involving many different locations on the body) or localized to several areas (e.g., neck and armpit).
The development of initial symptoms (e.g., fever, malaise, headache, weakness, etc.) marks the beginning of the prodromal period where the person could become contagious.
Following the prodrome, lesions will develop in the mouth and on the body. The characteristic rash associated with monkeypox lesions involve the following:
Lesions progress through several stages before falling off. A person is contagious from the onset of the enanthem through the scab stage.
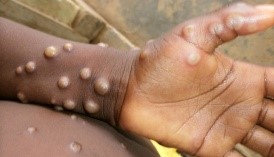
- Lesions are well circumscribed, deep seated, and often develop umbilication (resembles a dot on the top of the lesion). Lesions progression through specific sequential stages—macules, papules, vesicles, pustules, and scabs;
o This can sometimes be confused with other diseases that are more commonly encountered in clinical practice (e.g., secondary syphilis, herpes, and varicella zoster). Historically, sporadic accounts of patients co-infected with Monkeypox virus and other infectious agents (e.g., varicella zoster, syphilis) have been reported, so patients with
- Lesions are relatively the same size and same stage of development on a single site of the body (ex.: pustules on face or vesicles on legs)
- Fever before rash;
- Lymphadenopathy is common;
- Disseminated rash is centrifugal (more lesions on extremities, face);
- Lesions on palms, soles;
- Lesions are often described as painful until the healing phase when they become itchy (crusts).
Stages of Monkeypox Lesions
This information is from the CDC which outlines each stage after lesions begin to appear through the scab stage. After the rash is resolved, patients will have pitted scars and/or areas of lighter or darker skin may remain after scabs have fallen off.
Once all scabs have fallen off a person is no longer contagious.
Stage | Stage Duration | Characteristics of Lesions |
Enanthem | The first lesions to develop are on the tongue and in the mouth. | |
Macules | 1−2 days | Following the enanthem, a macular rash appears on the skin, starting on the face and spreading to the arms and legs and then to the hands and feet, including the palms and soles.
|
Papules | 1−2 days | By the third day of rash, lesions have progressed from macular (flat) to papular (raised). |
Vesicles | 1−2 days | By the fourth to fifth day, lesions have become vesicular (raised and filled with clear fluid). |
Pustules | 5−7 days | By the sixth to seventh day, lesions have become pustular (filled with opaque fluid) – sharply raised, usually round, and firm to the touch (deep seated).
|
Scabs | 7−14 days | By the end of the second week, pustules have crusted and scabbed over.
|
Monitoring Exposures
First, educate yourself, coworkers with direct patient contact, and your patients regarding how to prevent becoming infected with this virus.
- Avoid close, skin-to-skin contact with people who have a rash that looks like monkeypox.
o Do not touch the rash or scabs of a person with monkeypox.
o Do not kiss, hug, cuddle or have sex with someone with monkeypox.
- Avoid contact with objects and materials that a person with monkeypox has used.
o Do not share eating utensils or cups with a person with monkeypox.
o Do not handle or touch the bedding, towels, or clothing of a person with monkeypox.
- Wash your hands often with soap and water or use an alcohol-based hand sanitizer, especially before eating or touching your face and after you use the bathroom.
How can a person lower their risk during sex?
- The CDC provides educational advice on this topic – which is a little too explicit for this article. Click Here for monkeypox sexual health advice.
Utilize the CDC “Exposure Risk Assessment.”
The CDC provides health care professionals with information to determine the degree of exposure from High, Intermediate, Low/Uncertain to No Risk. This information is found by scrolling down on this CDC webpage:
Any healthcare worker who has cared for a monkeypox patient should be alert to the development of symptoms that could suggest monkeypox infection, especially within the 21-day period after the last date of care, and should notify infection control, occupational health, and the health department to be guided about a medical evaluation.
Considerations for Monkeypox Vaccination
The CDC recommends vaccination for people who have been exposed to monkeypox and people who are at higher risk of being exposed to monkeypox. On July 28, 2022, the Centers for Disease Control (CDC) updated Monkeypox Vaccination information on their website.
Two vaccines may be used for the prevention of Monkeypox virus infection:
- JYNNEOS (also known as Imvamune or Imvanex), licensed (or approved) by the U.S. Food and Drug Administration (FDA) for the prevention of Monkeypox virus infection, and
- ACAM2000, licensed (or approved) by FDA for use against smallpox and made available for use against monkeypox under an Expanded Access Investigational New Drug application.
ACAM2000 vaccine should not be used in people who have certain health conditions, such as a weakened immune system, skin conditions like eczema or other exfoliative skin conditions, or pregnancy.
As of August 2022, the CDC doesn't have data available on the effectiveness of these vaccines in the current outbreak.
Coding Monkeypox Tests & Vaccines
Human monkeypox and monkeypox infection diagnosis coding is under Chapter 1 code: Certain infectious and parasitic diseases (A00-B99) in ICD-10-CM and confirmed cases are coded B04 as follows:
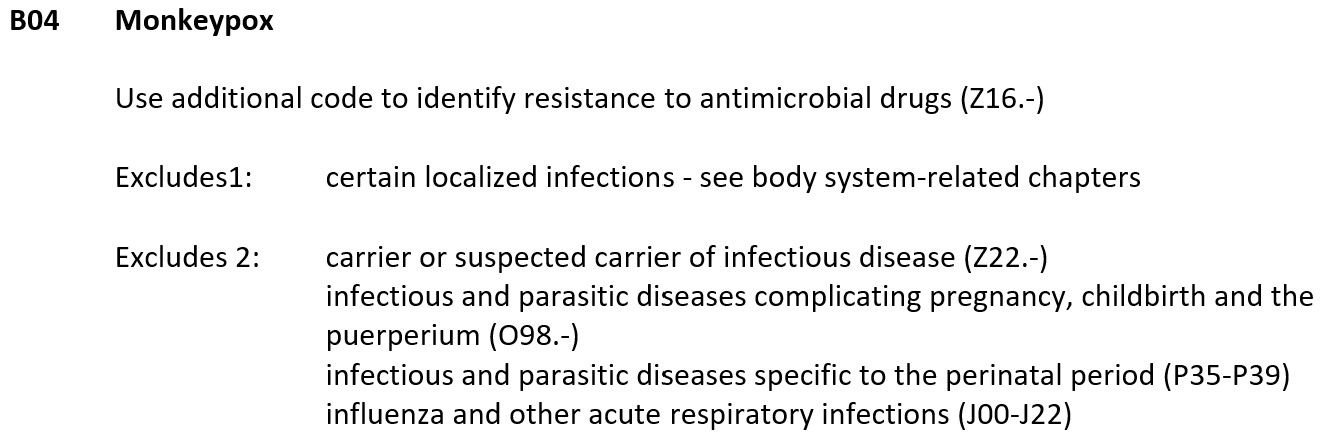
ICD-10-CM B04 is grouped within Diagnostic Related Group(s) (MS-DRG v39.0):
- 865 Viral illness with mcc
- 866 Viral illness without mcc
New Monkeypox Testing and Vaccine Codes
The American Medical Association (AMA), in response to the emergent nature of the public health concern surrounding monkeypox, has added three new Current Procedural Terminology (CPT®) codes specific to monkeypox.
The CPT® Panel approved one code, 87593, to report the laboratory diagnostic testing for the orthopoxvirus and two new vaccine product codes: 90611 and 90622, for the smallpox and monkeypox combined vaccine (JYNNEOS) and the traditional smallpox vaccine (ACAM2000), respectively.
The AMA expedited publication of these new codes to the AMA website at https://www.ama-assn.org/practice-management/cpt/orthopoxvirus-and-monkeypox-coding-guidance. These codes are effective immediately for use in reporting the laboratory test for the orthopoxvirus and vaccine administration utilizing these vaccine products.
- The AMA reminds coders that these codes do not appear in the CPT 2022 code set; however, they will be included in the CPT 2023 code set in the Microbiology subsection of the Pathology and Laboratory section and in the Vaccines, Toxoids subsection of the Medicine section, respectively.
87593 Infectious agent detection by nucleic acid (DNA or RNA); orthopoxvirus (e.g., monkeypox virus, cowpox virus, vaccinia virus), amplified probe technique, each
- Reporting code 87593 will help streamline the tracking of and reimbursement for testing services related to monkeypox in the United States.
90611 Smallpox and monkeypox vaccine, attenuated vaccinia virus, live, nonreplicating, preservative free, 0.5 mL dosage, suspension, for subcutaneous injection
90622 Vaccinia (smallpox) virus vaccine, live, lyophilized, 0.3 mL dosage, for percutaneous use
To meet the needs of the Centers for Disease Control and Prevention (CDC) safety-monitoring programs and to track the specific testing performed, it is imperative the appropriate code is listed on claim forms.
More information regarding monkeypox is available from the CDC at:
https://www.cdc.gov/poxvirus/monkeypox/index.html.
Conclusion
This virus is likely to continue spreading not only in the United States, but globally. As the disease progresses, more clinical and coding information will be available. At the time this article was written (August 2022), the United States was leading in the number of cases world-wide.
Please utilize the hyperlinks in this article to stay informed of new clinical and coding information. Also consider online training as a compliance auditor, a clinical appeals specialist and/or outpatient revenue cycle management through the American Institute of Healthcare Compliance.

