Written by Gabriella Neff, RHIA, CHA, CHC, CHRC, CHPC
This past year, in 2024, revisions were made to clarify hospital guidelines related to informed consent specifically addressing UIEs (unconsented intimate exams) to patients while under anesthesia. This article addresses how these privacy rights extend beyond rules designated under HIPAA and States passing rules banning unauthorized pelvic exams.
Revised Guidance for Teaching Hospitals and Medical Schools on Informed Consent
Privacy rights extend beyond the rules On April 1, 2024, the Centers for Medicare & Medicaid Services (CMS) released revisions and clarifications to the Hospital Interpretive Guidelines for Informed Consent simultaneously with a letter by the Department of Health and Human Services (HHS) to address medical professionals performing Unconsented Intimate Exams (UIEs), particularly on patients under anesthesia.[1],[2] UIEs are training and education-related examinations, including, but not limited to, pelvic, breast, prostate, and rectal examinations. These revisions resulted from recent articles, media reports, and concerns from nurses, some physicians, and medical students opposing these exams.[3],[4] These examinations have been known “to occur over the past thirty (30) years, for training and diagnostic purposes, mostly during gynecological surgeries but also during prostate examinations and abdominal surgeries.” [5]
In the letter, HHS reminded providers that the Office of Civil Rights (OCR) investigates complaints of impermissible use and disclosure of a patient’s Protected Health Information (PHI) in violation of the HIPAA Privacy Rule (Health Insurance Portability and Accountability Act), including scenarios where the patient may be unconscious during a medical procedure. OCR recently issued an FAQ focusing on this right.[6] The letter also notes that obtaining informed consent for sensitive examinations is the standard of care and that OCR will continue to focus on provider compliance with HIPAA and proper informed consent.
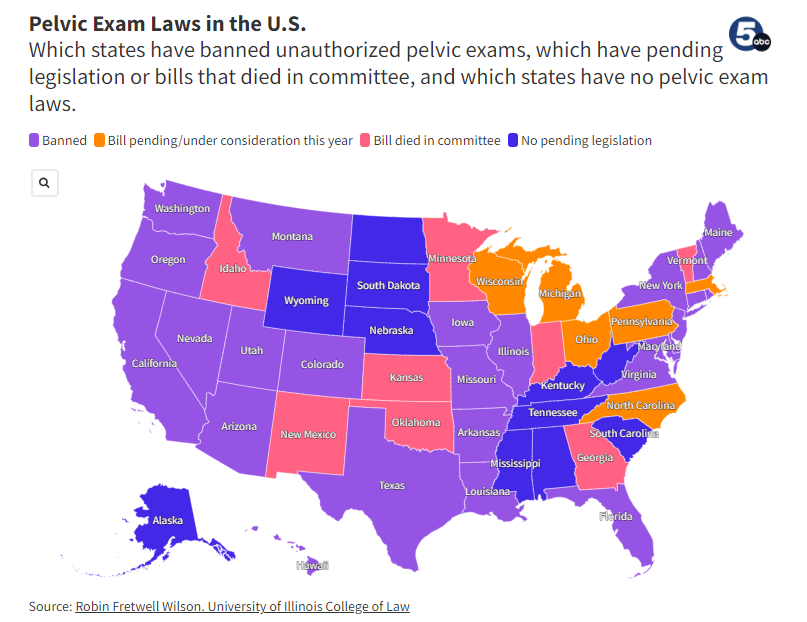
Many state regulators have legalized protections echoing this as well. As of November 2023, twenty-five (25) states passed legislation banning unauthorized pelvic exams. [7]
Informed consent is the process where a provider educates a patient about the risks, benefits, and alternatives of a procedure or intervention, which ultimately results in the patient’s agreement or refusal of the procedure or intervention. It further includes the patient's participation in the development of his/her plan of care during and after discharge from the hospital and providing consent to, or refusal of, medical or surgical interventions, including the right to refuse consent for sensitive examinations conducted for teaching purposes.
“Informed consent is the law and essential to maintaining trust in the patient-provider relationship and respecting patients’ autonomy.” [8] Requirements related to informed consent for hospitals may be found in the Hospital Conditions of Participation (CoPs):
- The Patient’s Rights CoP at 42 CFR 482.13(b)(2);
- The Medical Record Services CoP at 482.24(c)(4)(v);
- The Surgical Services CoP at 482.51(b)(2) [9]
- The State Operations Manual Appendix A, tag A-0955 – A properly executed informed consent.
CMS stated that they revised the interpretive guidance in the State Operations Manual Appendix A for hospitals at tag A-0955 under the section entitled “Properly Executed and Well-designed Informed Consent Form Examples” and under the section entitled “Hospital’s Policy and Process for Informed Consent. The revision is below in red:
“Whether physicians other than the operating practitioner, including, but not limited to, residents, medical, advanced practice providers (such as nurse practitioners and physician assistants), and other applicable students, will be performing important tasks related to the surgery, or examinations or invasive procedures for educational and training purposes, in accordance with the hospital’s policies. Important surgical tasks include opening and closing, dissecting tissue, removing tissue, harvesting grafts, transplanting tissue, administering anesthesia, implanting devices, and placing invasive lines. Examinations or invasive procedures conducted for educational and training purposes include but are not limited to, breast, pelvic, prostate, and rectal examinations, as well as others specified under state law.”[10]
Teaching hospitals and medical schools must understand the expectations outlined by CMS and take proactive steps to transform their institutional culture. Teaching hospitals need to establish guidelines addressing this matter, while medical schools should revise their curriculum to encourage a culture where healthcare providers and trainees consistently obtain and appropriately document informed consent from patients before conducting any sensitive examinations.
About the Author
Gabriella Neff, RHIA, CHA, CHC, CHRC, CHPC is a Research Compliance Officer for H. Lee Moffit Cancer Center and also serves as a Board Member for the American Institute of Healthcare Compliance.
Reference
[1] CMS Revisions and Clarifications to Hospital Interpretive Guidelines for Informed Consent. April 1, 2024. Retrieved from https://www.cms.gov/files/document/qso-24-10-hospitals.pdf
[2] HHS Letter to the nation’s teaching hospitals and medical schools. April 1, 2024. https://www.hhs.gov/about/news/2024/04/01/letter-to-the-nations-teaching-hospitals-and-medical-schools.html
[3] Friesen P, Wilson RF, Kim S, Goedken J. Consent for Intimate Exams on Unconscious Patients: Sharpening Legislative Efforts. Hastings Cent Rep. 2022 Jan;52(1):28-31. doi: 10.1002/hast.1337. PMID: 35143067
[4] Wilson RF. Unauthorized practice: teaching pelvic examination on women under anesthesia. J Am Med Womens Assoc (1972). 2003 Fall;58(4):217-20; discussion 221-2. PMID: 14640251
[5] Bruce L. A Pot Ignored Boils On: Sustained Calls for Explicit Consent of Intimate Medical Exams. HEC Forum. 2020 Jun;32(2):125-145. doi: 10.1007/s10730-020-09399-4. PMID: 32152870; PMCID: PMC7223770
[6] HHS. FAQ. Retrieved from https://www.hhs.gov/hipaa/for-professionals/faq/can-an-individual-restrict-who-has-access-to-their-protected-health-information-during-a-medical-procedure/index.html
[8] HHS Letter to the nation’s teaching hospitals and medical schools. April 1, 2024. https://www.hhs.gov/about/news/2024/04/01/letter-to-the-nations-teaching-hospitals-and-medical-schools.html
[9] Hospital Conditions of Participation. April 3, 2024. Retrieved from https://www.ecfr.gov/current/title-42/chapter-IV/subchapter-G/part-482?toc=1
[10] CMS State Operations Manual. Appendix A. – Survey Protocol. Regulations and Interpretive Guidelines for Hospitals. Retrieved from https://www.cms.gov/Regulations-and-Guidance/Guidance/Manuals/downloads/som107ap_a_hospitals.pdf
Copyright © 2024 American Institute of Healthcare Compliance All Rights Reserved
